Answer:
The pH of solution is 2.88 .
Step-by-step explanation:
The reaction is :
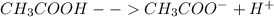
We know,
 for this reaction is =
for this reaction is =
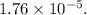
Also, since volume of water is 1 L.
Therefore, molarity of solution is equal to number of moles.
Also,
![K_a=([CH_3COO^-][H^+])/([CH_3COOH])](https://img.qammunity.org/2021/formulas/chemistry/college/ae94dtkjtl66tek45x24kx2b5zeccfcs90.png)
Let, amount of
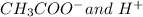 produce is x.
produce is x.
So,
![K_a=([x][x])/([0.1])\\1.76* 10^(-5)=([x][x])/([0.1])](https://img.qammunity.org/2021/formulas/chemistry/college/3lrhc3acagt8nofvbibgr6lrsc8cim399l.png)
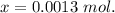
We know,
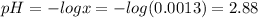
Therefore, pH of solution is 2.88 .
Hence, this is the required solution.