Answer:
Volume.
Step-by-step explanation:
Boyle’s law experiment :
This is also known as Mariotte's law or in the other words it is also known as Boyle–Mariotte law.
This law tell us about the variation of gas pressure when the volume of the gas changes at the constant temperature.According to this law the abslute pressure is inversely proportional to the volume .
We can say that
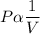
Where P=Pressure
V=Volume
PV = K
K=Constant
When volume decrease then the pressure of the gas will increase.
That is why the answer is "Volume".