Answer:
Frequency,

Step-by-step explanation:
It is given that, the electron moves in a circular orbit of radius 0.053 nm around a stationary proton. The electric force acting on the electron is balanced by the centripetal force as :
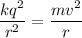
v is the speed of electron
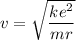
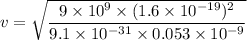

The speed of electron is given by :
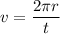
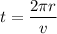
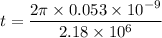
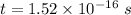
We know that the number of revolutions per second is called frequency of electron. It is given by :
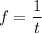
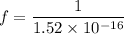

So, the total number of revolutions per second make by the electron is
 . Hence, this is required solution.
. Hence, this is required solution.