Step-by-step explanation:
According to the Einstein law, it is known that
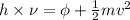
where, h = energy of light
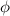 = work function
= work function
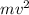 = kinetic energy of electron
= kinetic energy of electron
It is given that the value of
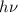 is
is
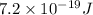 . And,
. And,
1 eV =
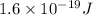
Here,
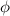 for titanium is 4.33 eV
for titanium is 4.33 eV
=
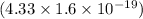 J
J
=
 J
J
(a) First of all, kinetic energy will be calculated as follows.
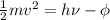
=
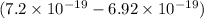 J
J
=
 J
J
It is known that mass of electrons is equal to
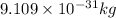 .
.
Therefore,
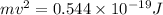
and,
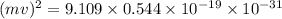
=

mv =

Now, the relation between wavelength and mv is as follows.
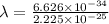
=
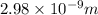
Therefore, the wavelength of the ejected electrons is
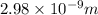 .
.
(b) It is known that relation between energy and wavelength is as follows.
E =
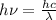
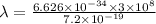
=
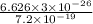
=
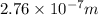
Hence, the wavelength of the ejected electrons is
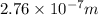 .
.
(c) For iron surface,
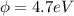
=
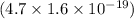 J
J
=
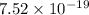 J
J
Here, the value of
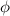 is more than the value of UV light source. Hence, we need a shorter wavelength light as we know that,
is more than the value of UV light source. Hence, we need a shorter wavelength light as we know that,
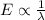
Therefore, lesser will be the wavelength higher will be the energy.