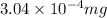The question is incomplete, here is the complete question:
In the Minnesota Department of Health set a health risk limit for methanol in groundwater of 4.00 μg/L. Suppose an analytical chemist receives a sample of groundwater with a measured volume of 76.0 mL.
Calculate the maximum mass in milligrams of methanol which the chemist could measure in this sample and still certify that the groundwater from which it came met Minnesota Department of Health standards. Be sure your answer has the correct number of significant digits.
Answer: The risk limit of methanol in the given amount of methanol is
Step-by-step explanation:
We are given:
Risk limit for methanol in groundwater = 4.00 μg/L = 0.004 mg/L (Conversion factor:
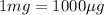 )
)
Volume of groundwater that is to be measured = 76.0 mL
We know that:
1 L = 1000 mL
Applying unitary method:
In 1000 mL of groundwater, the risk limit of methanol is 0.004 mg
So, in 76.0 mL of groundwater, the risk limit of methanol will be =
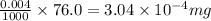
Hence, the risk limit of methanol in the given amount of methanol is