The theoretical mass of
 is 110.
is 110.
Step-by-step explanation:
Mass of one atom of calcium = 40 .
Mass of one atom of chlorine=35.5 .
Hence , two atoms would be
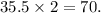
Therefore, mass of calcium chloride would be net sum both constituent atoms
=
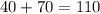
So the theoretical mass of
 is 110.
is 110.
The theoretical mass is the measure of item coming about because of an ideal chemical reaction, and in this manner not equivalent to the sum you'll really get from a response in the lab. It is the measure of item coming about because of an ideal chemical reaction, and therefore not equivalent to the sum you'll really get from a response in the lab.