Answer:
The answer to your question is m = 3.2
Step-by-step explanation:
Molality is defined as the number of moles of a solute dissolved in a mass of solvent (kg).
Data
moles of solute = 43.6
mass of solvent = 13.5 kg
Formula
Molality =
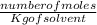
Substitution
Molality =
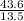
Simplification and result
Molality = 3.2