Answer:
c. 10°C
Step-by-step explanation:
Given that: A slice of pizza has 500 kcal.
That is to say Q = 500kcal
If the pizza was burned and all the heat were used to warm a 50-L container of cold water,
what would be the approximate increase in the temperature of the water?
We were also being told to note that; A liter of cold water weighs about 1 kg.
i.e 50-L container weighs about 50kg (mass)
∴ Since;
Q= 500kcal,
m = 50kg; &
the specific heact (c) = 1
we can find the approximate increase in the temperature of the water by using the formula:
Q = mcΔT
ΔT =
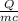
ΔT =
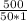
ΔT = 10°C