Answer:
a) The number density is 3.623 × 10⁻³
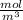
The mass of the atmosphere is 1.3 × 10²²Kg
b) The pressure is 10⁻²⁰ Millimeter of mercury
c) The mass mixing ratio is 0.0107
The partial pressure of ethane is 0.01114 Pa
Yes it is condensable because it boiling point is -88.5 C which is equivalent to 184.5 K i.e is adding 273 to -88.5C and the temperature of the atmosphere is 37 K.
Step-by-step explanation:
The explanation is on the first and second uploaded image