Step-by-step explanation:
Chemical reaction equation for the given reaction is as follows.
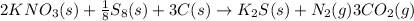
Therefore, we will calculate the number of moles of
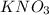 as follows.
as follows.
No. of moles =
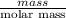
=
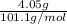
= 0.04 mol
Number of moles of nitrogen gas formed is calculated as follows.
No. of moles =
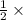
=
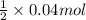
= 0.02 mol
Mass of
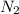 gas formed will be calculated as follows.
gas formed will be calculated as follows.
No. of moles × Molar mass of
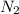
=
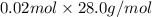
= 0.56 g
Now, the number of moles of
 formed is as follows.
formed is as follows.
=
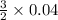
= 0.06 mol
Hence, mass of
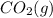 formed will be as follows.
formed will be as follows.
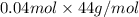
= 1.76 g
Volume of
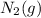 is calculated as follows.
is calculated as follows.
Volume =
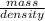
=
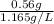
= 0.48 L
And, volume of
 is calculated as follows.
is calculated as follows.
Volume =
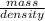
=
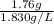
= 0.96 L
Let us assume that the volume of solids are negligible. Therefore, total volume will be as follows.
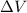 = (0.48 L + 0.96 L)
= (0.48 L + 0.96 L)
= 1.44 L
Relation between work, pressure and volume is as follows.
w = -
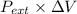
= -
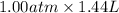
= -1.44 atm L
As 1 tm L = 101.3 J. So, convert 1.44 atm L into joules as follows.
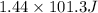
= 145.87 J
Thus, we can conclude that the given gases will do 145.87 J of work.