The given question is incomplete. The complete question is as follows.
Citrate synthase catalyzes the reaction
Oxaloacetate + acetyl-CoA
 citrate + HS-CoA
citrate + HS-CoA
The standard free energy change for the reaction is -31.5 kJ*mol^-1
( a) Calculate the equilibrium constant for this reaction a 37degrees C
Step-by-step explanation:
(a). It is known that , relation between change in free energy (
 ) of a reaction and equilibrium constant (K) is as follows.
) of a reaction and equilibrium constant (K) is as follows.
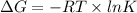
where, T = temperature in Kelvin
The given data is as follows.
T = 310 K,
 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J)
Now, putting the given values into the above formula as follows.
ln K =
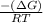
=
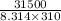
ln K = 12.22
K = antilog (12.22)
=

Therefore, we can conclude that value of equilibrium constant for the given reaction is
 .
.