Austin uses 435.714 milliliters of solution B to get a resulting mixture that has 244 milliliters of pure alcohol.
Explanation:
Step 1; Assume solution B has 1x ml of liquid in it. As solution A has three times that of solution B, it has 3x ml of liquid.
Step 2; In solution A there is 12% of alcohol and solution B has 20% alcohol. So alcohol content in solution A is 12% of 3x = 0.12 x 3x and the alcohol content in solution B is 20% of x= 0.20 x 1x.
Step 3; Use this formula
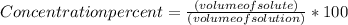
here Volume of solute is the total of solution A and B= (0.12 x 3x) + (0.20 x 1x) which equals 0.56x. The volume of solution is 3x + 1x which equals 4x. Substituting numerator and denominators we get a volume of 14%.
Step 4;This 14% is the concentration of alcohol in the mixture of solution A and solution B which is given as 244 ml. If 14% is 244 ml then 100% equals 1742.857 milliliters. The total of solution A and B equal 4x which is 1742.857 milliliters. So we get solution A has 1307.1428 milliliters and solution B has 435.71428 milliliters of liquid.