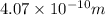Answer: The spacing between the crystal planes is
Step-by-step explanation:
To calculate the spacing between the crystal planes, we use the equation given by Bragg, which is:
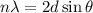
where,
n = order of diffraction = 2
 = wavelength of the light =
= wavelength of the light =
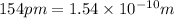 (Conversion factor:
(Conversion factor:
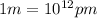 )
)
d = spacing between the crystal planes = ?
 = angle of diffraction = 22.20°
= angle of diffraction = 22.20°
Putting values in above equation, we get:
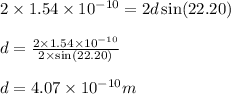
Hence, the spacing between the crystal planes is