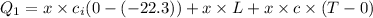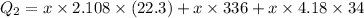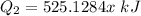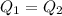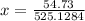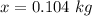Answer:
Step-by-step explanation:
Given
mass of water
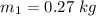
Temperature of water
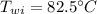
Initial Temperature of ice
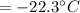
Final temperature of system
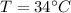
specific heat of water
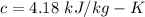
specific heat of ice
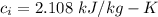
Latent heat of ice
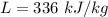
Heat loss by Water is equal to heat gained by ice
Heat loss by water
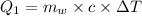
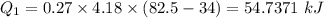
Heat gained by ice