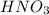Answer:
0.0078 L of the stock solution is required to make up 1.00 L of 0.13 M
Step-by-step explanation:
According to laws of equivalence,
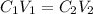
where,
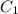 and
and
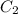 are initial and final concentration respectively.
are initial and final concentration respectively.
 and
and
 are initial and final volume respectively.
are initial and final volume respectively.
Here,
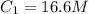 ,
,
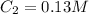 and
and
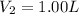
So,
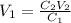
or,
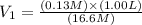
or,
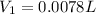
Hence 0.0078 L of the stock solution is required to make up 1.00 L of 0.13 M