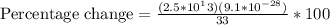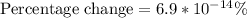The concept required to solve this problem is quantization of charge.
First the number of electrons will be calculated and then the total mass of the charge.
With these data it will be possible to calculate the percentage of load in the mass.
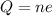
Here Q is the charge, n is the number of electrons and e is the charge on the electron
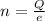
Replacing,
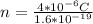
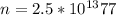
According to the quantization of charge the charge is defined as product of the number of electron and the charge on the electron
The total mass of the charge is
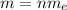
Here,
m = Mass of the charge
n = Number of electrons
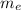 = Mass of the electron
= Mass of the electron
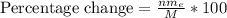
Replacing we have