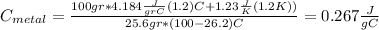Answer:
a)
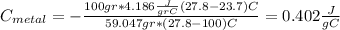
b)
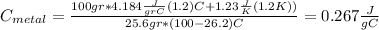
Step-by-step explanation:
Part a
For this case we have the following data:
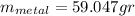 the mass of the metal
the mass of the metal
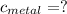 Is the value that we need to find
Is the value that we need to find
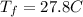 represent the final temperature of equilibrium for the metal and the water
represent the final temperature of equilibrium for the metal and the water
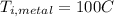 represent the initial temperature for the metal
represent the initial temperature for the metal
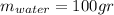 since the density is 1g/ml
since the density is 1g/ml
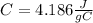 the specific heat for the liquid water
the specific heat for the liquid water
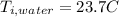 the initial temperature for the water
the initial temperature for the water
For this case we have this equation:
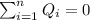 if we have balance then we have this:
if we have balance then we have this:
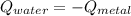
And if we replace the formulas for heat we got:
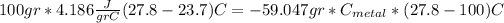
And if we solve for
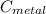 we got:
we got:
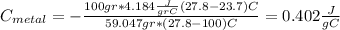
Part b
For this case we have the following balance:
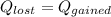
On this case the metal loss heat and this heat is gained by the water and the calorimeter, so we have this:

And if we replace the info given we have this:
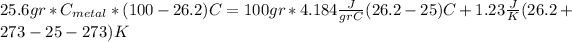
And if we solve for
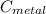 we got:
we got: