Answer:
Ratio of rates of effusion of He to Ne is 2.245
Step-by-step explanation:
According to Graham's law rate of effusion is inversely proportional to square root of molar mass of a gas
So,
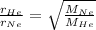
where,
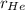 and
and
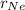 are rate of effusion of He and Ne respectively.
are rate of effusion of He and Ne respectively.
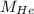 and
and
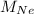 are molar mass of He and Ne respectively.
are molar mass of He and Ne respectively.
Molar mass of He = 4.003 g/mol
Molar mass of Ne = 20.18 g/mol
So,
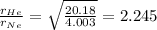
So, ratio of rates of effusion of He to Ne is 2.245