Answer: The mass of nitrogen gas that must be reacted is 1.4 grams
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
Given mass of ammonia = 1.7 g
Molar mass of ammonia = 17 g/mol
Putting values in equation 1, we get:
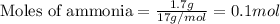
The chemical equation for the production of ammonia follows:
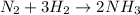
By Stoichiometry of the reaction:
2 moles of ammonia is produced by 1 mole of nitrogen gas
So, 0.1 moles of ammonia will be produced from
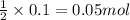 of nitrogen gas
of nitrogen gas
Now, calculating the mass of nitrogen gas from equation 1, we get:
Molar mass of nitrogen gas = 28 g/mol
Moles of nitrogen gas = 0.05 moles
Putting values in equation 1, we get:
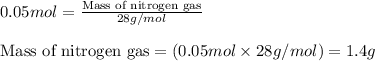
Hence, the mass of nitrogen gas that must be reacted is 1.4 grams