Answer:
a) 1.165 × 10⁻⁸ N b)- 1.165 × 10⁻⁸ N
Step-by-step explanation:
Using Coulomb's law
F(attraction) =
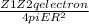
where
R = sum of the distance between the centers of charges = sum of ionic radii = 0.079 nm + 0.120nm = 0.199 nm = 0.199 × 10⁻⁹ m
Z₁ = valency of Mg²⁺ = 2
Z₂ = valency of F ⁻ = - 1
qelectron = charge on electron =1.062 × 10⁻19 C
E = permitivity of free space = 8.85 × 10 ⁻¹² C²/ Nm²
Fa= (1×2× (1.602 × 10⁻¹⁹)²) / (4× 3.142 × 8.85 × 10⁻¹² × (0.199 × 10⁻⁹)²) = 1.165 × 10⁻⁸ N
b) At equilibrium F of repulsion = - F of attraction = - 1.165 × 10⁻⁸ N