Answer:
The value of
 for xylene is 4.309°C/m.
for xylene is 4.309°C/m.
The molar mass of pentane using this data is 73.82 g/mol.
Step-by-step explanation:
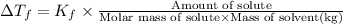
where,
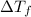 =depression in freezing point
=depression in freezing point
 = freezing point constant
= freezing point constant
we have :
1) freezing point constant for xylene =
 =?
=?
Mass of toluene = 0.193 g
Mass of xylene = 2.532 kg = 0.002532 kg ( 1 g =0.001 kg)
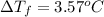
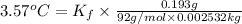
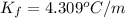
The value of
 for xylene is 4.309°C/m.
for xylene is 4.309°C/m.
2)
Mass of pentane = 0.123 g
molar mass of pentame= M
Mass of xylene = 2.493 g = 0.002493 kg
Freezing point Constant of xylene =
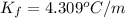
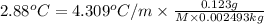
M = 73.82 g/mol
The molar mass of pentane using this data is 73.82 g/mol.