Answer : The mass of
 produced form the combustion is, 16.43 kg
produced form the combustion is, 16.43 kg
Explanation :
Density : It is defined as the mass contained per unit volume.
Formula used for density :
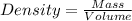
First we have to calculate the mass of octane.
Given :
Density of octane = 0.703 g/mL
Volume = 2.00 gallons = 7570 mL
conversion used : 1 gallon = 3785 mL
Now put all the given values in the above formula, we get:
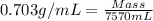
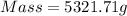
Now we have to calculate the moles of octane.
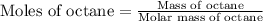
Molar mass of octane = 114 g/mole
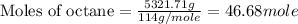
Now we have to calculate the moles of
 .
.
The balanced chemical combustion reaction of octane will be:
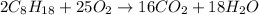
From the balanced chemical reaction we conclude that:
As, 2 moles of
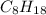 react to give 16 moles of
react to give 16 moles of

So, 46.68 moles of
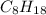 react to give
react to give
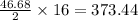 moles of
moles of

Now we have to calculate the mass of
 .
.
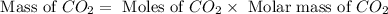
Molar mass of
 = 44 g/mol
= 44 g/mol
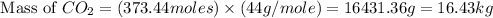
Thus, the mass of
 produced form the combustion is, 16.43 kg
produced form the combustion is, 16.43 kg