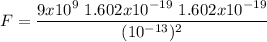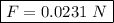Answer:
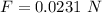
Step-by-step explanation:
Electrostatic Force
When two point charges
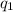 and
and
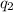 are placed at a distance d, they exert a force to each other whose magnitude can be computed by the Coulomb's formula
are placed at a distance d, they exert a force to each other whose magnitude can be computed by the Coulomb's formula
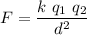
Where k is the constant of proportionality
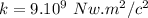
The information provided is
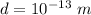
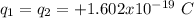
Computing the Coloumb force between them