Answer:
The equilibrium concentration of NH3 = 0.141 mol/L
Step-by-step explanation:
The balanced equation for the reaction is :
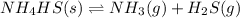
Here , you have to write the Kc carefully "Don't write SOLID substance in the expression of Kc"
Kc = equilibrium constant for concentration( Solid are not included in the expression)
So, Kc can be written as :
![Kc=[NH_(3)][H_(2)S]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/mnqlc48h81iyuldx0gutxq5fzaji6nki91.png)
Now , look at the equation ,
Let x moles are dissociated from it at equilibrium
[NH4HS](s) NH3 H2S
1 0.28 0 (Initial moles)
1-x x + 0.28 x (Moles at equilibrium)
Calculate the concentration at equilibrium :
Moles of NH3 = 0.28 mol
The concentration of NH3 is calculated using :
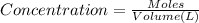
![[NH_(3)]=(0.28 +x)/(2)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/jyf1sqo3uqatf3tq5pts29xx51v6yvtc0r.png)
Similarly concentration of H2S
![[H_(2)S]=(x)/(2)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/ra93lkw25iv0l84017rebk9frilz66jjlu.png)
Put the value in given equation:
![Kc=[NH_(3)][H_(2)S]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/mnqlc48h81iyuldx0gutxq5fzaji6nki91.png)
Kc = 0.00016
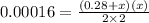
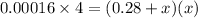
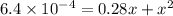
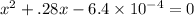
Use the formula of quadratic equation to solve the value of
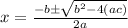
Here, b= 0.28 , c= -.00064 and a = 1
you get ,
x = 0.002267 mol/L
or
[NH3] = (x + 0.28)/2 = 0.002267 + 0.28
[NH3] = 0.2822/2 mol/L
[NH3] = 0.141 mol/L