Answer:
The numerical value of equilibrium constant is 0.0560
Step-by-step explanation:
Initial Concentration:
![[NH^3]= (3mol)/(1l)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/2lwu3u49aizmaf8vrhspdmwzyt7zi3b3l7.png) = 3M
= 3M
[
 ] = 1M
] = 1M
[
 ] = 2 M
] = 2 M
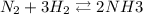
at the end, [
 ] = 1.96 M
] = 1.96 M
Thus the change is 3 - 1.96 = 1.04 M
Thus 1.04 moles of reacted
By stoichiometry, 1.04 moles (
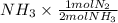 ) = 0.52 mol created (in addition to 1 mol already in vessel)
) = 0.52 mol created (in addition to 1 mol already in vessel)
By similar reasoning =
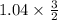 1.56 moles created
1.56 moles created
Final concentrations:
[
 ] = 1.96 M
] = 1.96 M
[
 ] = 0.52 + 1 = 1.52 M
] = 0.52 + 1 = 1.52 M
[
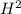 ] = 1.56 + 2 = 3.56 M
] = 1.56 + 2 = 3.56 M
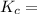
![([NH^3]^2)/(N^2[H^2]^3)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/uutru3vi8xdgs77esryl6vjvmppeazayog.png)
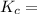
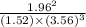
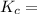 0.0560
0.0560
Therefore, the numerical value of equilibrium constant is 0.0560