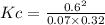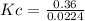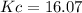Answer:
The equilibrium constant Kc for the reaction is :
16.07
Step-by-step explanation:
The balanced equation is :
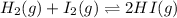
First ,
"At the equilibrium, 60% of the hydrogen gas had reacted"
This means the degree of dissociation = 60% = 0.6
Here we are denoting degree of dissociation by ="x" = 0.6
Now , consider the equation again,
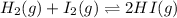
H2 I2 2HI
0.35 1.6 0 (Initial Concentration)
0.35(1 - x) 1.6(1 - x) 2x (At equilibrium)
0.35(1 - 0.6) 1.6(1 - 0.6) 2(0.6)
0.14 0.64 1.2
calculate the concentration of each:
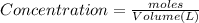
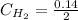
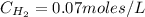
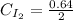
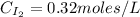
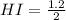
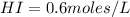
The equilibrium constant for this reaction "Kc" can be written as:
![Kc=([HI]^(2))/([H_(2)][I_(2)])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/9lkz82wjtny82lo1bf88p51qv3m0uz458c.png)