Answer:
The heat of vaporization in kJ/mol of methanol
= 3.48 kJ/mole
Step-by-step explanation:
Here,the heat of vaporization in kJ/mol is asked to calculate.This means it is asked to calculate heat required for 1 mole of methanol. Since the substance vaporizes , so this is called heat of vaporization.
Divide the thermal energy with the number of moles .
This is simple mathematics :
If 2.50 mol of liquid require = 8.70 kJ of energy
Then, 1 mole will need =
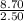
= 3.48 kJ/mole
Follow the units :
The heat of vaporization in kJ/mol of methanol is asked.
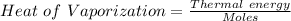
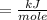
Unit = kJ /Mole