Answer:
The heat of vaporisation of methanol is "3.48 KJ/Mol"
Step-by-step explanation:
The amount of heat energy required to convert or transform 1 gram of liquid to vapour is called heat of vaporisation
When 8.7 KJ of heat energy is required to vaporize 2.5 mol of liquid methanol.
Hence, for 1 mol of liquid methanol, amount of heat energy required to evaporate the methanol is =
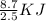
= 3.48 KJ
So, the heat of vaporization
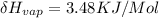
Therefore, the heat of vaporization of methanol is 3.48KJ/Mol