321.1 g of strontium phosphite will contain 0.76 moles of strontium phosphite in the beaker.
Step-by-step explanation:
It is known that 1 mole of any substance is equal to the molar mass of that substance. So for the case of strontium phosphite, the molar mass is found to be 420.8 g/mol.
This means 1 mole of Strontium phosphite will contain 420.8 g of strontium phosphite.
Then 1 g of strontium phosphite will have
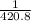 moles of strontium phosphite.
moles of strontium phosphite.
So, 321.1 g of strontium phosphite will contain
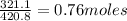
Thus, 321.1 g of strontium phosphite will contain 0.76 moles of strontium phosphite in the beaker.