Answer : The change in enthalpy of the coffee is negative.
Explanation :
Endothermic reaction : It is defined as the chemical reaction in which the energy is absorbed from the surrounding.
In the endothermic reaction, the energy of reactant are less than the energy of product.
Exothermic reaction : It is defined as the chemical reaction in which the energy is released into the surrounding.
In the exothermic reaction, the energy of reactant are more than the energy of product.
Enthalpy change : It is the difference between the energy of product and the reactant. It is represented as
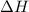 .
.
- When the system gains energy in the form of heat then the change in enthalpy is positive.
- When the system loses energy in the form of heat then the change in enthalpy is negative.
As per question, if hot coffee sits on your desktop and loses 50 kJ of energy during cooling, the change in enthalpy of the coffee is negative.
Hence, change in enthalpy of the coffee is negative.