The given question is incomplete. The complete question is as follows.
The value of Henry's law constant
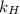 for oxygen in water at
for oxygen in water at
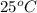 is
is
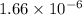 M/torr.
M/torr.
Calculate the solubility of oxygen in water at
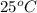 when the total external pressure is 1 atm and the mole fraction of oxygen in the air is 0.20 atm.
when the total external pressure is 1 atm and the mole fraction of oxygen in the air is 0.20 atm.
Step-by-step explanation:
Formula to calculate partial pressure of a gas is as follows.
Partial pressure of oxygen = mole fraction of oxygen x total pressure
Putting the given values into the above equation as follows.
=
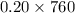 = 152 torr
= 152 torr
Therefore, solubilty (concentration) of oxygen in water will be calculated as follows.
Solubility = Henry's law constant x partial pressure of oxygen
=
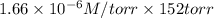
=
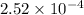 M
M
Thus, we can conclude that solubility of given oxygen is
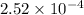 M.
M.