Answer: 2.38 grams of silver forms when 3.00g of zinc metal is placed in an aqueous solution containing 3.75g of silver nitrate.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 ....(1)
....(1)
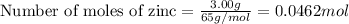
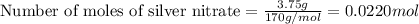
The chemical equation is:
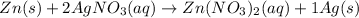
By stoichiometry of the reaction;
2 moles of silver nitrate reacts with 1 mole of zinc
Thus 0.0220 moles silver nitrate react with=
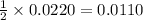 moles of zinc
moles of zinc
Thus silver nitrate will acts as limiting reagent and zinc acts as excess reagent.
2 moles of silver nitrate produces 2 mole of silver
Thus 0.0220 moles silver nitrate react with=
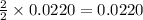 moles of silver
moles of silver
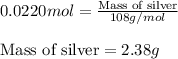
Thus 2.38 grams of silver forms when 3.00g of zinc metal is placed in an aqueous solution containing 3.75g of silver nitrate.