2 moles of NaOH dissolved in 1 litre of solution is the solution with more concentration.
Answer: Option A
Step-by-step explanation:
Concentration of solution is the measure of the amount of solute dissolved in the solvent of the solution. So this is measured using the molarity of the solution. Molarity is determined as the number of moles of the solute present in the given amount of solvent.
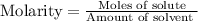
In this present case, the option A gives the molarity of 2 M as
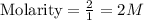
But the second option, mass of NaOH is given. So we have to determine the molarity. First we have to find the molar mass of NaOH. We know that 1 mole of NaOH will contain 40 g/mole.
1 g of NaOH = 40 g of NaOH
1 g of NaOH = 1/40 moles
So 2 g of NaOH will contain
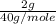 which is equal to 0.05 moles of NaOH.
which is equal to 0.05 moles of NaOH.
Thus, the molarity of 2 g of NaOH will be
Molarity =
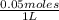 =0.05 M
=0.05 M
Thus, the option A is having higher concentration as the molarity is more for 2 moles of NaOH dissolved in 1 l of solution.