The higher concentrated solution is 0.250 mole LiCl dissolved in 250 ml of solution
( B).0.250mole of LiCl diluted in 250ml solution
Step-by-step explanation:
Procedure to find the intensity of the solution:
To find the intensity of the solution, we have to use the following formula
M1V1=M2V2
250mL of a 0.500 M aqueous solution of LiCl is diluted with water
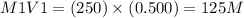
250 ml of a 0.250M aqueous solution of LiCl is diluted with water
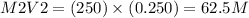
Thus the PH level of a solution increases from ascending order, Therefore the Solution with 62.5M is a higher concentration than that of the solution with 125M.