There are 0.0747×
 molecules in 0.0124 moles of
molecules in 0.0124 moles of
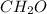 .
.
Step-by-step explanation:
We know that 1 mole of any element is equal to Avagadro's number of molecules present in that element. So in this case, it is stated that 0.0124 moles of
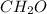 is present. Then the number of molecules will be obtained by multiplying the number of moles with Avagadro's number.
is present. Then the number of molecules will be obtained by multiplying the number of moles with Avagadro's number.
No. of molecules = No.of moles × Avagadro's number
No.of molecules = 0.0124×6.022×
 molecules =0.0747×
molecules =0.0747×
Thus, 0.0747×
 molecules of
molecules of
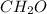 are present in 0.0124 moles of
are present in 0.0124 moles of
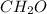 .
.