Dry ice sublimes into carbon dioxide gas. If the proper conditions are maintained and the system is closed, the dry ice and the carbon dioxide gas will eventually.
1 )become the same phase
2) reach equilibrium
3)same properties and composition throughout
4) both become same phase and reach equilibrium
Answer:
Under the the proper conditions maintained over the closed system , the dry ice and the carbon dioxide gas will eventually reach equilibrium.
Step-by-step explanation:
The reactions which do not go on completion and in which the reactant forms product and the products goes back to the reactants simultaneously are known as equilibrium reactions.
Equilibrium state is the state when reactants and products are present but the concentrations does not change with time.
For a chemical equilibrium reaction, equilibrium state is achieved when the rate of forward reaction becomes equals to rate of the backward reaction.
Under the the proper conditions maintained over the closed system , the dry ice and the carbon dioxide gas will eventually reach equilibrium.
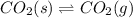
Amount of carbon dioxide changing from solid to gas will be equal to amount of carbon dioxide changing from gas to solid.