Answer:
The answer is 298.74K
Step-by-step explanation:
Ideal gas formula is following:
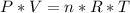
We are assuming that, except temperature and pressure, all other variables are constant. So if we define morning temperature and pressure as:
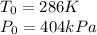
Then if we write the same formula for afternoon time the equation will be:
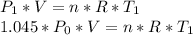
Then we find out that the must be correlation between morning and afternoon temperature as following:
