Answer:
The density of a material given is 1.55 g/mL
Step-by-step explanation:
Density = Mass per unit volume of the substance .
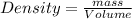
Initial Volume of water = 47.54 mL
Total volume = Volume of water + material = 50.78 mL
Volume of material = Total volume - volume of water
Volume of material = 50.78 - 47.54 = 3.24 mL
Volume of material = 3.24 mL
Mass of the material = 5.03 g
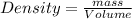
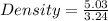
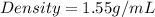
The density of a material given is 1.55 g/mL