Answer:
The volume of gas is 58.22 L which will occupy at 250.°C and 680 mm Hg pressure.
Step-by-step explanation:
Given:
Temperature = 27°C
To make it into kelvin = 27+273
= 300K
Pressure =740mm Hg
to make it in atm. =
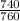
= 0.973atm
n = 2.3 moles
Volume of gas =?
As we know according to the formula
PV = nRT
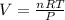
On substituting the values
=
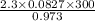
= 58.22 L
Therefore, 58.22L volume of gas will occupy at 250.°C and 680 mm Hg pressure.