Answer:
16.96 moles of sodium will needed to react with 4.24 moles of oxygen gas.
Step-by-step explanation:
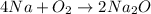
Moles of oxygen gas = 4.24 moles
According to reaction , 1 mole of oxygen gas reacts with 4 moles of sodium.
Then 4.24 moles of oxygen gas will react with :
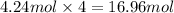 of sodium
of sodium
16.96 moles of sodium will needed to react with 4.24 moles of oxygen gas.