The given question is incomplete. The complete question is as follows.
Consider two gases, A and B, that are in a container at room temperature.
What effect will the following change have on the rate of the reaction between these gases?
The temperature is decreased at a constant volume.
Step-by-step explanation:
Relation between kinetic energy and temperature is as follows.
K.E =
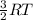
As kinetic energy is directly proportional to temperature. Therefore, more is the increase in temperature more will be the movement of molecules of a substance.
Hence, more will be the kinetic energy which will lead to more number of collisions. Therefore, rate of reaction will also increase.
But when we decrease the temperature by keeping the volume constant there this will also lead to decrease in kinetic energy of the molecules. Hence, rate of reaction will also decrease.
Thus, we can conclude that when temperature is decreased at a constant volume then rate of the reaction between these gases also decreases.