Answer:
The longest wavelength observed in the Balmer series of the H atom spectrum is 656.3 nm.
The shortest wavelength observed in the Balmer series of the H atom spectrum is 364.6 nm.
Step-by-step explanation:
Using Rydberg's Equation:
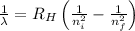
Where,
 = Wavelength of radiation
= Wavelength of radiation
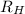 = Rydberg's Constant
= Rydberg's Constant
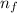 = Higher energy level
= Higher energy level
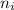 = Lower energy level
= Lower energy level
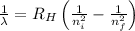
For wavelength to be longest, energy would be minimum, i.e the electron will jump from third level to second level :
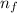 = Higher energy level =
= Higher energy level =
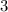
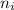 = Lower energy level = 2 (Balmer series)
= Lower energy level = 2 (Balmer series)
Putting the values, in above equation, we get
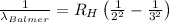
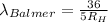
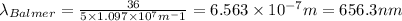
The longest wavelength observed in the Balmer series of the H atom spectrum is 656.3 nm.
For wavelength to be shortest, energy would be maximum, i.e the electron will from infinite level to second level. :
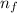 = Higher energy level =
= Higher energy level =
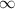
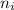 = Lower energy level = 2 (Balmer series)
= Lower energy level = 2 (Balmer series)
Putting the values, in above equation, we get
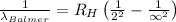
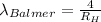
The shortest wavelength observed in the Balmer series of the H atom spectrum is 364.6 nm.