Answer:
Atomic mass of 35.5 g/mol is of chlorine.
Atomic mass of 89.02 g/mol is of Yttrium.
Ytterium(III) chloride is the correct name for
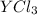 .
.
1.835 grams of
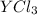 can be prepared.
can be prepared.
Step-by-step explanation:
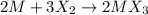
Moles of
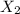 =n
=n
Number of moleules of
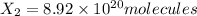
1 mole =
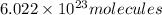
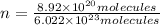
n = 0.001481 mole
Mass of
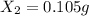
Molar mass of
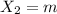
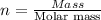
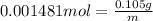
m = 71 g/mol
Atomic mass of X =
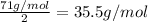
Atomic mass of 35.5 g/mol is of chlorine.
The compound MX3 consists of 54.47% X by mass:
Molar mass of compound = M'
Percentage of element in compound :
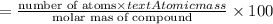
X:
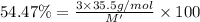
M' = 195.52 g/mol
Molar mass of compound = M'
M' = 1 × (atomic mass of M)+ 3 × (atomic mass of X)
195.52 g/mol = atomic mass of M + 3 × (35.5 g/mol)
Atomic mass of M = 89.02 g/mol
Atomic mass of 89.02 g/mol is of Yttrium.
Ytterium(III) chloride is the correct name for
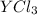 .
.
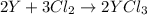
Moles of Yttrium =
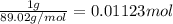
Moles of chlorine gas=
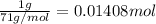
According to reaction, 3 moles of chlorine reacts with 2 moles of Y.
Then 0.01408 moles of chlorine gas will :
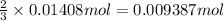 of Y.
of Y.
This means that chlorine is in limiting amount., So, amount of yttrium (III) chloride will depend upon amount of chlorine gas.
According to reaction , 3 moles of chlorine gives 2 moles of
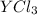
Then 0.01408 moles of chlorine will give :
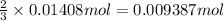 of
of
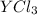
Mass of 0.009387 moles of
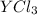 :
:
0.009387 mol × 195.52 g/mol = 1.835 g
1.835 grams of
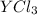 can be prepared.
can be prepared.