Answer: The Cu-63 isotope has the highest percent natural abundance
Step-by-step explanation:
Average atomic mass is defined as the sum of masses of each isotope each multiplied by their natural fractional abundance.
Formula used to calculate average atomic mass follows:
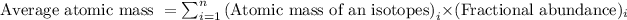
We are given:
Two isotopes of Copper, which are Cu-63 and Cu-65
Average atomic mass of copper = 63.54 amu
As, the average atomic mass of copper is closer to the mass of Cu-63 isotope. This means that the relative abundance of this isotope is the highest as compared to the other isotope.
Percentage abundance of Cu-63 isotope = 69.2%
Percentage abundance of Cu-65 isotope = 30.8 %
Hence, the Cu-63 isotope has the highest percent natural abundance