Isotopic notation of phosphorus-29 is
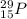
Step-by-step explanation:
- Isotopes are atoms with equal number of protons but different number of neutrons at its nucleus.
- The isotopic notation for any element can be written as
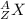 where X represents the chemical symbol ,A represents mass number and Z represents atomic number
where X represents the chemical symbol ,A represents mass number and Z represents atomic number - The atomic number for phosphorus is 15
- Isotope's mass number for phosphorus-29 is 29
- The chemical symbol for phosphorus is P
- Hence Isotopic notation is
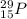
- Number of neutrons = 29-15 =14
- Number of protons=atomic number =15