Corrected question:
Bromine-88 is radioactive and has a half life of 16.3seconds. What percentage of a sample would be left after 35seconds? Round your answer to 2 significant digits.
Answer:
23% (3 significant digits)
Step-by-step explanation:
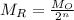
where
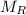 is mass remaining
is mass remaining
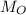 is original mass
is original mass
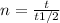
t1/2= 16.3s , t=35s
n =35/16.3 =2.147
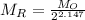
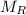 =
=
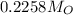
0.2258*100% =22.5%
≈23%