The question is incomplete, here is the complete question:
A chemist prepares a solution of aluminum chloride by measuring out of 11. g aluminum chloride into a 50. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's aluminum chloride solution.
Answer: The concentration of aluminium chloride solution is 1.65 mol/L
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
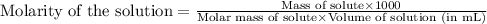
We are given:
Given mass of aluminium chloride = 11. g
Molar mass of aluminium chloride = 133.34 g/mol
Volume of solution = 50. mL
Putting values in above equation, we get:
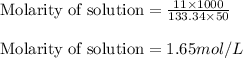
Hence, the concentration of aluminium chloride solution is 1.65 mol/L