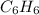Answer:
The molecular formula =
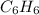
Step-by-step explanation:
Given that:
Mass of compound, m = 0.145 g
Temperature = 200 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T = (200 + 273.15) K = 473.15 K
V = 97.2 mL = 0.0972 L
Pressure = 0.74 atm
Considering,
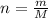
Using ideal gas equation as:
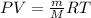
where,
P is the pressure
V is the volume
m is the mass of the gas
M is the molar mass of the gas
T is the temperature
R is Gas constant having value = 0.0821 L.atm/K.mol
Applying the values in the above equation as:-
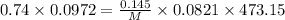
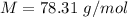
The empirical formula is =
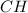
Molecular formulas is the actual number of atoms of each element in the compound while empirical formulas is the simplest or reduced ratio of the elements in the compound.
Thus,
Molecular mass = n × Empirical mass
Where, n is any positive number from 1, 2, 3...
Mass from the Empirical formula = 12 + 1 = 13 g/mol
Molar mass = 78.31 g/mol
So,
Molecular mass = n × Empirical mass
78.31 = n × 13
⇒ n ≅ 6
The molecular formula =