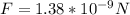Answer:
Step-by-step explanation:
According to Coulomb's law, the magnitude of the electric force between two point charges is directly proportional to the product of the magnitude of both charges and inversely proportional to the square of the distance that separates them:
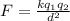
Here k is the Coulomb constant. In this case, we have
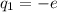 ,
,
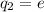 and
and
 . Replacing the values:
. Replacing the values:
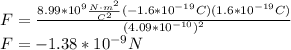
The negative sign indicates that it is an attractive force. So, the magnitude of the electric force is: