Molecular mass of Cobalt(II) Bromide = 218.7412 g/mol .
Step-by-step explanation:
Percent composition by element:
The procedure to find the Molecular mass of Cobalt(II) Bromide:
The symbol of the Cobalt is Co.
The symbol of the Bromate is Br.
The Element cobalt have the Atomic Mass of 58.933200 with 1 number of atom.
The Element Bromine have the Atomic Mass of 79.904 with 2 number of atoms.
Molecular weight calculation:
To find the molecular mass we have to sum both of the Atomic masses:
For Cobalt(II) Bromide: We have to multiple the Atomic mass of bromate with 2 and then sum the atomic masses
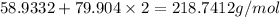
Molecular mass of Cobalt(II) Bromide = 218.7412 g/mol